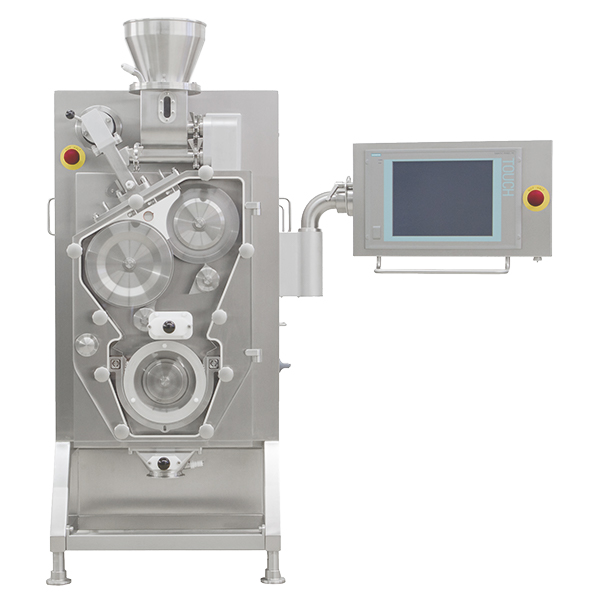
Importance of equipment reliability Successfully manufacturing a drug product requires people, proper equipment, and materials. It’s the classic three-legged stool analogy. Even with well-trained staff and high-quality materials, if the equipment does not function as expected, or is not reliable, the final product can never be cost-effectively produced. Product quality will likely be compromised...