Download our eBook and learn how a robust CLD strategy can help biologics developers anticipate and overcome challenges and streamline production.
Proprietary host cell-line and high-expression vector provide superb production efficiency
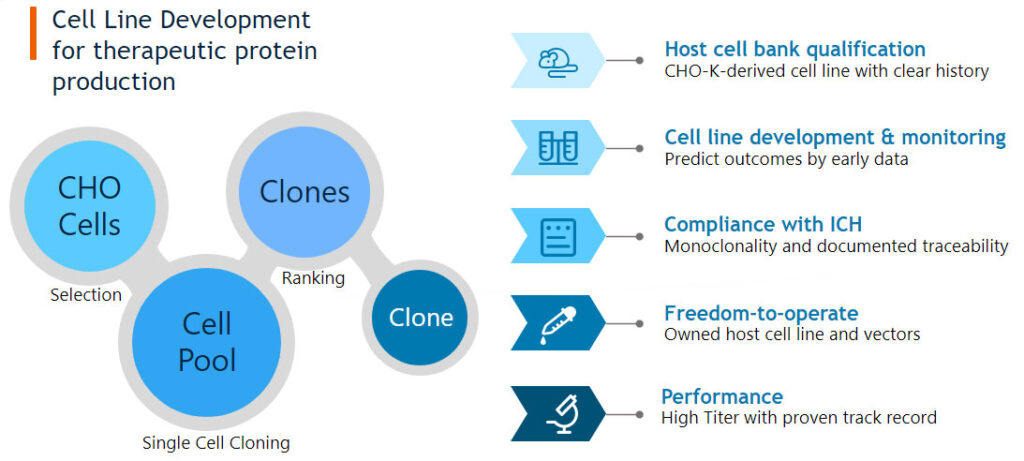
Bora generates high performance production cell lines under a target product profile using our proprietary CHO expression technologies. With the implementation of a state-of-the-art single cell printer and high-resolution imager we can ensure monoclonality and reduce production time of single cell cloning.
High-Throughput Clone Screening
Bora’s state-of-the-art single-cell printer technology provides an outstanding single-cell cloning efficiency which enables screening of thousands of clones at a time. With this cost-effective platform, we can offer the best path to selecting the optimal, high-performance clone for all client’s projects, while proving monoclonality.
A scalable platform technology to achieve ideal protein product quality and yield
Bora’s scalable platform technologies for cell culture and purification provide optimal protein product qualities and yield. We develop processes in 3L and 50L, and scale up to 200L, 500L or 1,000L (2x500L) for use in non-clinical, clinical trials and commercial production.


In-depth medium analysis and sustained development
Cell culture process development ensures robust processes suitable for cGMP clinical and commercial manufacturing and process validation. Our process development includes clone screening in bioreactors, media and feed development, defining optimal ranges for bioreactor operation and control parameters.
Process Development, optimization and tech transfer
Bora Biologics offers comprehensive purification process development services, including process optimization, tech transfer, clinical research, and process characterization. Specializing in mAb and mAb derivatives, Bora optimizes impurity removal and provide analytical support. Bora’s expertise ensures seamless Tech Transfer with track records. Our experts also generate tailored material for preclinical studies and employ risk-based parameter assessment and statistical analysis for robust process characterization.



