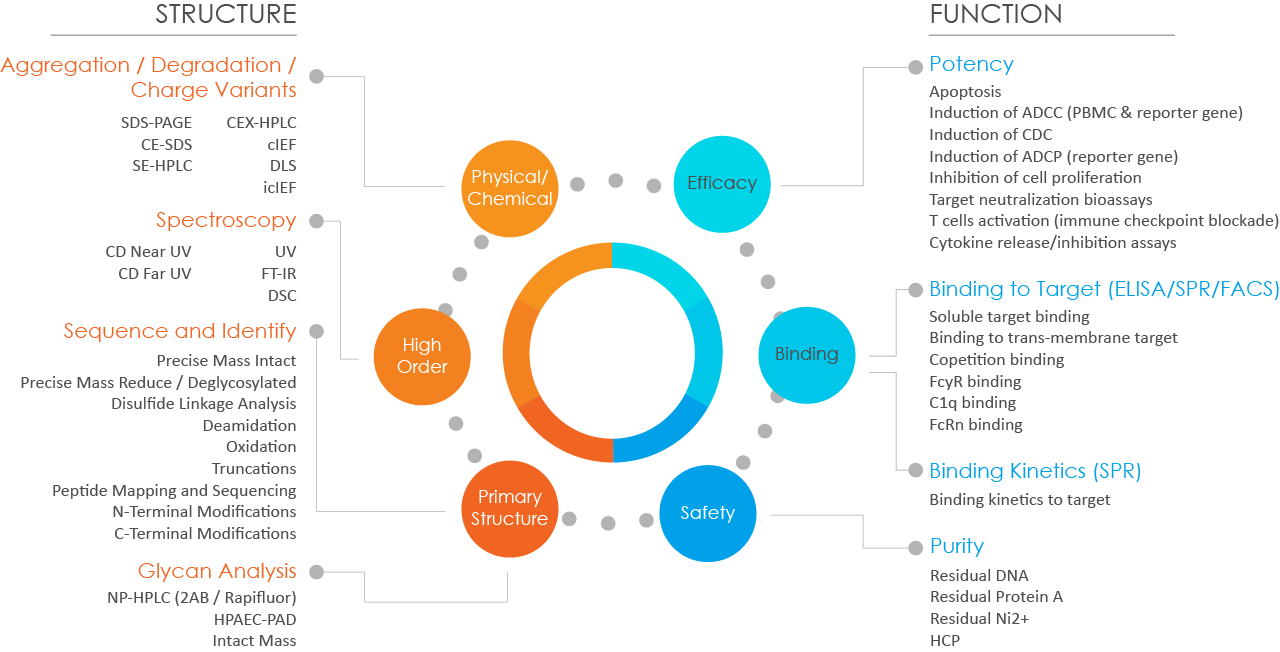
Full Range of Analytical Development Capabilities
Read our special article to explore the importance of well-designed analytical testing throughout biopharmaceutical development, emphasizing strategies for success in meeting regulatory requirements and optimizing processes.

Formulation Development for DS and DP
From tox to pre-clinical and clinical development, Bora Biologics provides full-range formulation services to fulfill clients’ needs. We specialize in developing liquid or lyo formulations in vial or prefilled syringe for different administration routes including intravenous, subcutaneous, intravitreal, and inhalation. Lyophilization cycle development and scale up can also be done in-house.
Our team carries out phase appropriate formulation development approach by DoE to ensure candidates move forward on designated timeline with an established drug substance and drug product stability program and justification for product shelf-life.
Bora utilizes a QbD approach to drug product process development and characterization to support your project from development toward commercial launch.


