Importance of good flow
Good powder flow is particularly important when manufacturing tablets and capsules because rapid and accurate filling of the tablet die or capsule body is essential to achieve consistent weights, and thus, a consistent drug dose.
When powdered active pharmaceutical ingredients (API) possess poor flow characteristics, they present a handling challenge during the manufacturing process and can be a potential source for segregation in powder blends. Roller compaction helps to mitigate these and other challenges caused by poorly behaving APIs in formulation by improving the content uniformity and flow of powder blends.
A dry, continuous process
Roller compaction is a dry process and therefore better suited for moisture and heat-sensitive material. Furthermore, roller compaction is a continuous process, enabling single-instrument configurations that accommodate a greater range of batch sizes compared to wet granulation.
When deciding if roller compaction is the best approach for a project, consider first the appropriateness of the formulation for manufacturing using the method. Typically, a roller compaction formulation consists of:
- API
- Filler (e.g., microcrystalline cellulose (MCC) and or lactose)
- Disintegrant (e.g., croscarmellose sodium, (CROS-povidone)
- Lubricant (e.g., magnesium stearate, stearic acid)
- Glidant (e.g., silicone dioxide) to reduce potential roller surface friction and facilitate powder flow)
Formulations possessing drug loadings less than 60% wt are generally found to be amenable for roller compaction.
Careful characterization provides insight into challenges
Table 1 details the material characteristics that comprise an ideal candidate for roller compaction. The more carefully the materials’ attributes are assessed, the better insight we will have into potential manufacturing challenges. For instance, blends with low bulk density or poor flow properties may not efficiently pass through the rollers during compaction. Insufficient compactability may indicate a potential for tablet friability during the compaction of resultant granules.
Table 1 Properties of an Ideal Roller Compaction Material
| Property | Value | Target value |
| Compactability | At 0.7 solid fraction | Tensile strength >1 MPa |
| Loss of compactabilitya | At <0.9 solid fraction | Tensile strength >1.7 MPa |
| Bulk density | At >0.14 solid fraction | >0.2g/mLb |
| Wall friction | Angle of wall friction | <20 degrees c |
| Flow assessment |
Flow function coefficient Carr’s Index |
>4c <35% |
| Solid-state properties |
Melting point Glass transition (Tg) Loss of crystallinity during compaction |
>90℃ >90℃ None |
| Particle size for content uniformity | Blend potency | Meets Rohr’s criteria |
aLoss of compactability defined as the compactability remaining in granules.
bUSP ,616. Method I—Measurement in a graduated cylinder.
cShear cell method, measured over the range 1 to 5 KPa.
Adapted from Leane et al. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm Dev Technol 2015;20(1):12~21.
Adhesion challenges and mitigation strategies
The propensity for a material to adhere to surfaces (i.e., roll surface) is another important trait to consider. Material adhesion challenges, for example, are typically associated with APIs rather than excipients. When the material is observed to adhere to a surface, proper excipient selection is often the first-line strategy during early development stages to mitigate adhesion challenges.
Mitigation tactics typically included modifying the lubricant selection or level. If this doesn’t address adhesion issues it might indicate the need to change the API form, particle size or particle morphology. If powder adhesion still cannot be properly rectified, alternative granulation techniques, such as wet granulation methods, and possibly even suspensions are suggested.
Variable gap and temperature issues
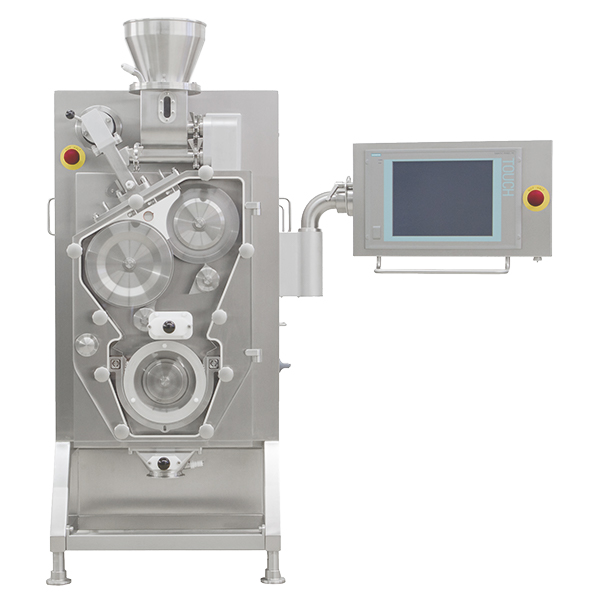
Bora’s roller compaction capabilities are delivered by the industry standard Gerteis MINI-PACTOR ®. The system is equipped with an inclined press and variable gap rollers system, which helps avoid temperature issues and allows for more precise dosing of powders. The precision delivered by the variable gap control also helps minimize fluctuations in the ribbon’s solid fraction caused by variations in powder flow. An integrated size reduction/milling technology the system offers the following capacities:
- Range: 10 g up to 100kg/h
- Press Force: 1 – 20 kN/cm
- Roll gap: 1 – 6 mm
- Roll Speed: 1 – 30 rpm
- Granulator speed: 1-150 rpm
- Granulator angle: 0 – 1800° msec
Roller selection critical to process outcomes
Roller surface design is also understood to impact powder flow through the roller compactor. Patterned roll surfaces (i.e., knurled, serrated) assist in “grabbing” powder and facilitating powder flow between the rolls. Roll surface design may also be a critical factor with powders that have a high tendency to stick or adhere to surfaces.
The finish of the roll surface can affect roller compaction properties as well. Design elements such as the nip and entry angles during compaction, and thus the cumulative pressure applied to the powder during the process may disrupt desired physicochemical properties. It is therefore best practice to select the rolls as early as possible to provide consistency throughout the development process.
It is also recommended to evaluate different roll designs and combinations — smooth upper and lower rollers versus a smooth upper roller and a knurled lower roller for example. When conducting preliminary experiments, typical roller gaps (2.0mm-3.0 mm) and 3 to 4 different roll pressure values should be evaluated.
Feed screw performance is another excellent indicator of powder flow performance. For example, if the powder is not effectively drawn through the rolls and back pressure is generated, then the load (i.e., torque) on the feed screw may increase.
Key operating parameters and product attributes
The key parameters typically monitored during roller compaction operations are roll gap, feed screw speed, and roll pressure because they impact ribbon/ granulation quality the most. As mentioned, Bora’s roller compactor features a floating roller technology to minimize fluctuations in ribbon solid fraction.
This allows roll gap and feed screw speed to fluctuate continuously throughout roller compaction operation in response to variations in powder flow. With the feedback loop controlling feed screw speed, the average of these values remains consistent once the operation reaches a steady state. Additional process parameters, such as roller speed and mill screen size, may also impact ribbon/granules properties but are generally considered to be “fixed” parameters for a given run — because they do not fluctuate during compaction.
Roll speed selection is often based on throughput. Changes to the roller speed may change the ribbon tensile strength for a given roller pressure because the dwell time (i.e., the duration that pressure is applied to the powder by the rolls) is impacted.
Mill screen size will influence granule particle size, flow, and compactability, and a change in mill screen size may necessitate changes to other process parameters. Thus, an appropriate selection of these “fixed” parameters should be conducted early in the development stage.
Table 2 offers key operating parameters and corresponding ribbon and granule attributes.
Table 2 Key product and operating parameters
| Key operating parameters | Ribbon attributes | Granule attributes |
| Roller pressure | Solid fraction/porosity | Particle size distribution |
| Feed pressure (i.e., feed screw speed) | Tensile strength | Compactability |
|
Roll speed Roll gap | Thickness | Flow |
A go-to technology ready to meet today’s processing challenges
With roller compaction, both formulators and processors have more options to prepare the drug substance and improve things like solubility and bioavailability. API compounds can present chemistries that are hard to process into OSD forms.
Roller compaction, in the right hands, can provide a cost-effective solution and the flexibility developers need to help formulations go with the flow and fly through development and into the hands of patients faster.
Get in touch with one of our experts to discuss your next OSD project.