In this blog, we explore why and how the technique of extrusion-spheronization offers pharmaceutical companies a robust, proven way to manufacture today’s most complex therapies. We also discuss the key considerations for drug developers who are looking to select a contract manufacturer as a partner to deliver this technique.
The Right Choice for Encapsulated Solid Dose Formulations
In the right hands, with the right capabilities, extrusion spheronization is a well-understood process ready to manufacture today’s and tomorrow’s complex and potent capsule-delivered solid dose therapeutics.
 Extrusion-spheronization’s reputation as a versatile and economic manufacturing technique is well-earned. It’s viable for most of today’s-solid dose development paths and is proving to be an effective formulation and manufacturing technology for everything from New Molecular Entities (NMEs), Abbreviated New Drug Applications (ANDAs), life-cycle management (LCM) extensions, to OTC marketing strategies.
Extrusion-spheronization’s reputation as a versatile and economic manufacturing technique is well-earned. It’s viable for most of today’s-solid dose development paths and is proving to be an effective formulation and manufacturing technology for everything from New Molecular Entities (NMEs), Abbreviated New Drug Applications (ANDAs), life-cycle management (LCM) extensions, to OTC marketing strategies.
Because of its broad drug-engineering utility, extrusion-spheronization can also support emerging 505(B)(2) development pathways. This includes tailoring compounds and developing fixed-dose combinations (FDCs) to create new best-in-class, patient preferred products.
A Versatile Technique for Today’s Complex, Encapsulated Medications
To accomplish therapeutic effect, and deliver API payloads with precision, pharmaceutical formulators are leveraging a number of drug delivery tactics, including fixed-dose combinations (FDCs) and combined, modified-release, single-capsule forms.
Spheronized drug particles offer a variety of versatile attributes that drug developers can leverage to control the dosing and support the therapeutic performance of their encapsulated medications. This can help them to achieve patient preferred goals like reducing side effects or reducing the frequency of dosing.
Better Dose Control Equals Better Outcomes
Extrusion-spheronization allows for a broad range of controls to manage API release. Modified release formulations are effective for drug substances that have a narrow therapeutic index or need to support better dose compliance. Multi-unit particle systems for example are proving to demonstrate higher levels of desired reproducible pharmacokinetic and pharmacodynamic (PK and PD) behaviours (including insoluble APIs) resulting in improved therapeutic effect in patients.
Sphere of Influence in a Capsule
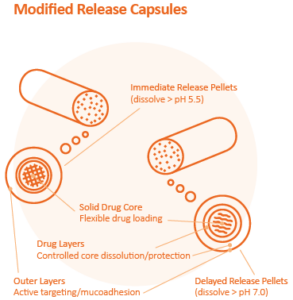
As patient preferences manifest themselves across the life-sciences industry, tailoring medications to better suit a particular patient population’s special needs has become a priority. But where the movement is being realized most profoundly is in drug engineering, with much of pharma pursuing fundamental improvements to the therapeutic formulation, functionality, and form of solid dose drugs.
Advanced Technologies Unlocking Extrusion-Spheronization’s Inherent Potential
Extrusion-spheronization is a mature methodology but the process and the equipment integral to it have benefited with time. The technology itself has seen steady improvement (along with a host of incumbent tertiary systems) and consequently some contract manufacturers have been better prepared to manufacture complex encapsulated solid dose drugs with this technique than others.
Quality in context
In the context of pharmaceutical quality, CGMP thinking in extrusion-spheronization means incorporating Quality-by-Design (QbD) chemistry, state-of-the-art mixing, extruding and spheronizing equipment. The process requires both experience and the investment operationally to do it well and meet all commercial, financial, and regulatory expectations.
Pharmaceutical drug development organizations are relying more and more on its contract manufacturing partners to make complex drugs with increasingly complex processes. For those seeking more strategic outsourcing relationships, it is now more important than ever to match drug strategy as closely as possible to the expertise and technical capabilities of your manufacturing partners.
