GxP is a general term used to describe the quality guidelines and regulations applied in the pharmaceutical industry. GxP is the abbreviation of “Good x Practice”. The “x” in GxP stands for the field the guidelines and regulations applied to.
Here are examples of GxPs often seen in the pharmaceutical industry:
- GMP – Good Manufacturing Practice
- GLP – Good Laboratory Practice
- GDP – Good Distribution Practice
- GCP – Good Clinical Practice
Although different regulations are applied in different countries/regions, the fundamental concept of GxP is similar across the globe. In the US, GxP regulations are defined in the Code of Federal Regulations (CFR) Title 21. In the EU, GxP regulations are defined in EudraLex. In many countries, the PIC/S (Pharmaceutical Inspection Co-operation Scheme) GMP and the ICH (The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use) guidelines are adapted.
GxP and Product Quality
The origin of the GxP was to target low quality drug products that affect public health. Several public health incidents, including the 1941 Sulfathiazole Tablets Disaster and the 1960 thalidomide tragedy, prompted the establishment of the modern GxP guidance.
GxP is the minimal quality requirements used by the regulatory agencies to regulate pharmaceutical companies. However, GxP should not only be considered as the “minimal” requirements because it provides a fundamental framework for continuous quality improvement. By applying the quality tools described in the GxP guidelines, a company can greatly improve its product quality as it expands its knowledge base. Regulatory agencies also constantly renew their thinking on different GxP topics as the technologies applied in drug product manufacturing progress.
Why should Pharmaceutical Manufacturers Follow GxP?
First and foremost, GxP is the law. Unlike other quality systems which a company can choose to follow or not, GxP is mandatory for producing and distributing pharmaceutical products. One may face regulatory actions for not following GxP and that includes fines, forfeiture, recalls, and other legal actions.
Not following GxP also results in bad quality systems and bad product quality. Many issues, such as product Out Of Specifications (OOS), may arise due to bad quality systems.
Here are some downsides of not following GxP:
- Getting observations (483) in regulatory inspections
- Getting warning letters
- Import alert
- Delay of application approval
- Product recalls
- Product rejections
- Product customer complaints
By following GxP, a company can not only reduce the risk of facing issues from the regulatory agencies but can also benefit from better quality system:
- Higher production yield
- Fewer product rejections
- Stable product supply
- Fewer customer complaints
Working with a Pharmaceutical Manufacturer that follows GxP Guidelines
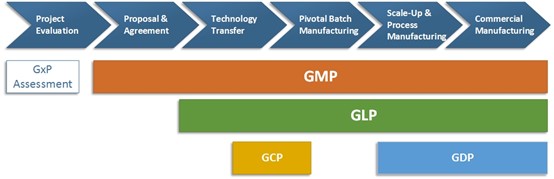
Suppose you are a drug product license holder and you want to work with a CDMO to develop and manufacture your drug product, here are some aspects to consider:
- Project Evaluation: When you approach a CDMO, it is important to understand if the CDMO follows GxP. Choosing a CDMO with good regulatory inspection history will reduce the risk caused by compliance issues.
- Proposal & Agreement: During this stage, the license holder and the CDMO should follow the GxP guidance to establish a formal contractual relationship. Responsibilities related to quality activities should be documented in a Quality Agreement.
- Technology Transfer: Although technology transfer is sometimes seen as part of the R&D process in a CDMO, the GxP concept can be applied during this stage. Tools like Quality by Design (QbD) described in GxP guidelines are very useful to facilitate technology transfer and build up a knowledge base for the products.
- Pivotal Batch Manufacturing: Although a pivotal batch may not be distributed to the markets, GxP should be followed during the manufacturing of a pivotal batch because the data generated for the batch are going to be submitted to the regulatory agencies. GMP and GLP should be followed for manufacturing and testing in the CDMO. If a clinical trial is required, GCP should be followed and there is usually a Contract Research Organization (CRO) involved in this process.
- Scale-Up & Process Manufacturing: Scale-up and process validation stage is critical both to the compliance and business aspects. Several guidelines published by the regulatory agencies (for example, “Guidance for Industry: Process Validation: General Principles and Practices” by the USFDA) should be considered during this stage. If the product license owner and the CDMO use quality tools from the technology transfer stage, they should have adequate knowledge to quickly moving from pivotal (small) batch scale to commercial (large) batch scale.
- Commercial Manufacturing: Quality systems fully complying with GxP should be in place for commercial manufacturing. In addition to GMP and GLP mentioned in the previous stages, companies should also follow GDP to distribute products to the markets.
In all stages of the process, there may be inspections by the regulatory agencies. The inspections could be routine inspections or pre-approval inspections. By maintaining a good state of GxP compliance, a company should pass any kind of inspection without critical observations.
Pharmaceutical companies should apply GxPs and the best scientific technology in the quality management system. Pharmaceutical companies should not place patients at risk due to the inadequate safety, quality, or efficacy of drug products. Understanding and following GxPs is a good way to avoid crucial business risk due to regulatory actions and greatly improve product quality.
