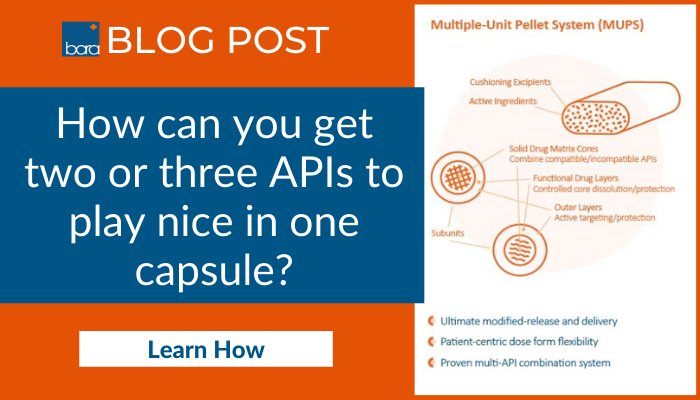
Multiple-unit pellet system (MUPS) can offer a solution to complex dose control
Oral solid dose (OSD) delivery remains the most popular route for administering drugs. Market forces are prompting pharmaceutical companies to pursue a broad array of OSD development strategies to make their active pharmaceutical ingredients (APIs) and formulations therapeutically effective and patient friendly.
One choice that is growing in popularity to control dose frequency and side-effects are compressed, tablet form multiple-unit pellet systems (MUPS). A versatile technology integrating proven pelletization and tablet delivery modalities. MUPS delivery offers new modified-release profiles and the dosing precision pharma needs
for its advanced formulations and potent, combined APIs. It’s a great way to get multiple APIs to “play nice” together. This technology is not new and has been used in the food and chemicals business for many years with many commercially available products, e.g. slow release fertilizers and flavorings.
Helping Complex Drugs Become Easier to Swallow
According to Nihad Al-Hashimi, et al, in the paper “Oral Modified Release Multiple-Unit Particulate Systems: Compressed Pellets, Microparticles and Nanoparticles” , no matter the particular nomenclature, “MUPS” offers process efficiencies and manufacturing economies and by design, patient-friendly attributes compared to capsules.[1]
Offering comprehensive value and utility, Multi-Unit Pellet System technologies can help manage API release control and modification more economically relative to formulation work. This is because they can be made with higher processing throughput and therefore, are potentially less expensive to produce commercially compared to other oral solid dose form products.
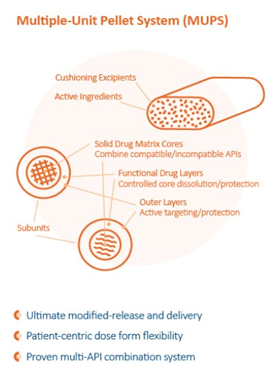 Multi-Particle Formulations Require Particularly Solid Pellet and Tablet Capabilities
Multi-Particle Formulations Require Particularly Solid Pellet and Tablet Capabilities
Although tablet manufacturing is generally well understood, it takes greater understanding and operational competency across a number of oral solids processing techniques to manufacture MUPS correctly. It also requires well-organized operational and quality teams to meet the demanding requirements of formulators, developers, and regulators.
But fundamental to the success of any MUPS delivery strategy is the ability to formulate, process, and manufacture the pellets that are at the core of the finished dose form’s functionality.
Integrating Multiple Oral Solid Dose Technologies in a Single Dose
Playing a central role in the functional performance of the dosage form is complex pelletizing and particle processing techniques. The two most popular capabilities for this type of drug form are Extrusion-Spheronization and Polymeric Core Coating.Decades of technical advancement in OSD formulation and tablet processing capabilities provide the basis on which high-performance MUPS are built. The diagram shows how a MUPS dose combines multiple components including APIs, excipients, and multi-functional pellets in a single dose form.
Extrusion-Spheronization Provides Powerful Pellets for Today’s Multi-Unit Pellet System Delivery
Depending on the API compounds and delivery strategies, formulators and processors can leverage the attributes of the popular pellet technology to create precision release profiles for what are often referred to as the API “reservoirs” within a MUPS.
However, Al-Hashimi notes that the reservoir pellets have to have sufficient physical properties to withstand the pressures of MUPS tablet compression. Similarly, the study explains pellets often require specialized polymeric coatings to control interactions between the tablet’s API/excipient formulation and the reservoir particles held in the oral solids matrix.
At the end of the day, specific attributes or limitations of the pellet technology relative to the interactions of materials, chemistry, and API payloads will ultimately determine the best particle process choice to meet drug product goals.
However, studies show extruded and spheronized API pellets have physical attributes that withstand tableting pressures better than particles made from coating inert cores.[1]
In the right hands with the right formulation, extrusion spheronization offers process reliability and broad utility and the flexibility to build the functionality developers want to achieve for their MUPS pellets. Extrusion-spheronization is a well-understood process that, executed correctly, offers the quality, efficiency required to manufacture today’s complex (including potent) OSD therapeutics.
Advanced Technologies Unlock Extrusion-Spheronization’s Inherent Drug Delivery Potential
Continuous improvement over time has helped manufacturers optimize the technology and associated processes for commercial applications.
The equipment itself has also seen steady improvement (along with a host of incumbent tertiary systems) including the addition of more precise process analytical tools. Consequently, some specialty manufacturers may be better prepared to manufacture complex encapsulated oral solid dose products with this technique than others.
Extrusion-Spheronization Provides an Optimal Processing Environment for MUPS
In the context of MUPS product and process quality, it is critical to offer CGMP tablet manufacturing integrated with optimized extrusion-spheronization capabilities. Incorporating Quality-by-Design (QbD) chemistry, state-of-the-art mixing, extruding, and advanced spheronizing equipment within a continuous-flow process, specialty manufacturers have to commit operationally to do it well and meet all commercial, financial and regulatory expectations.
Extrusion-spheronization’s reputation as a versatile and economic manufacturing technique is well-earned. It is viable for most of today’s sophisticated OSD development paths while proving effective as versatile formulation and manufacturing technology suitable for New Molecular Entities (NMEs), life-cycle management (LCM) patent extensions, and emerging OTC marketing strategies.
Increasingly pharmaceutical companies are relying on CDMOs to make their complex drugs because of their expertise and overall mastery of compliant, complex drug processing and manufacture. For those seeking more effective outsourcing relationships, and more efficient and economic development routes, it’s become critically important to match drug strategy as closely as possible to the expertise and technical capabilities of your outsourcing partners.
At Bora, we understand solving complex drug delivery challenges with MUPS with an experienced project team will help you get your product to market faster.