Equipment qualification is critical in the pharmaceutical industry. The equipment not only includes manufacturing equipment but testing and sampling equipment also to name a few. Even the slightest error or misuse can pose a costly risk and time delay for the pharmaceutical product owner and their manufacturer. So, what is equipment qualification? It is a series of inspections, tests, and assessments to ensure that a given piece of equipment is compliant and ensures reliable performance. Equipment validation is required to prove that a given piece of equipment does, on a consistent basis, what it is supposed to do. As a key component of quality assurance, equipment qualification is critical to consistently producing high-quality products.
Why is equipment qualification required?
First of all, qualification is required by regulatory authorities. FDA, EMA, MHRA, and WHO require that GMP (Good Manufacturing Practice) equipment used for manufacturing pharmaceutical drugs need to be qualified before released for their intended use. During the qualification process, a piece of equipment will be tested to prove that it meets the requirements of what a user has expected (user specification) and what the equipment is designed for (design/functional specification).
The three major stages of an equipment protocol qualification are:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
In the Specification group, the critical attributes for the equipment are User Requirement Specification (URS), Functional Requirement Specification (FRS), and Design Specification (DS).
Moreover, the qualification process needs to be documented. The auditor will visit the drug manufacturing site on a routine basis and documentation is certainly one of the main focuses of the audit. Those documents are the best way to prove that the equipment qualification was properly performed.
What are equipment qualification documents?
The following diagram depicts the relationship of different qualification documents with one another:
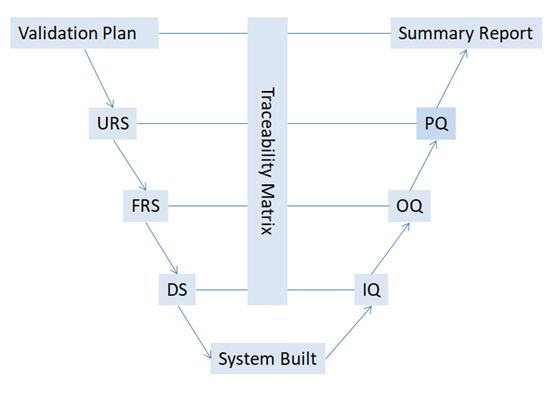
The documents required for equipment qualification include:
- Validation Plan (VP)
- User Requirement Specification (URS)
- Functional Requirement Specification (FRS)
- Design Specification (DS)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Traceability Matrix (TM)
- Summary Report
Those documents can be categorized into three groups:
- Specification
- Protocol
- Report
In the specification group, the written document includes the critical attributes for the equipment from the user’s and manufacturer’s aspects. User Requirement Specification (URS), Functional Requirement Specification (FRS), and Design Specification (DS) are in this group.
In the protocol group, the written procedures state how the qualification will be conducted. This may include the test units, test procedures, critical test parameters, operating ranges, sampling plan, and acceptance criteria. Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
In the report group, the written document reports the qualification activities and results and traces back to the test items with their specifications/ requirements Traceability Matrix, ™ and Summary Report.
VP is the validation plan that outlines the validation/qualification strategy.
What happens after equipment qualification is completed?
The summary report will determine if the equipment passes the qualification or not. Only equipment which passed qualification can be used for manufacturing. After a piece of equipment is “qualified,” the qualification status of that qualified equipment should be reviewed and the results can be used to determine if and when requalification is needed. Such review includes, at a minimum, calibration, preventive maintenance, deviations, and change history of the equipment. This review task will need to be maintained during the time the equipment is in use, and can only be finally completed when the equipment is inactivated or decommissioned.
Conclusion
Equipment qualification/validation is an absolute must to ensure that the equipment is installed, operates, and consistently performs to its purpose. The qualification documents provide evidence to an auditor so they can review and ensure the equipment is well-maintained to ensure compliance, reduces the risk of misuse and helps prevent future problems. Equipment qualification is key to ensure that the piece of equipment performs consistently and produces high-quality products throughout production.
