To submit a filing to the FDA, whether that be an NDA, ANDA, or 505(b)(2), a product owner must provide technical files containing a pharmaceutical product’s chemistry, manufacturing, and control (CMC), safety (nonclinical), and efficacy (clinical) testing results before gaining approval and market access. This includes analytical testing procedures for the drug substances and the finished product. These analytical testing methods must meet certain standards to ensure product safety and efficacy through the life of the product.
These standards of reliability and accuracy must be maintained through the site-to-site technology transfer of the product. One of the key elements of any technology transfer is to figure out the analytical scope of work to minimize site-to-site and lot-to-lot variability. For a contract manufacturer, one of the first steps in evaluating a technology transfer project is: do we need to perform analytical method verification or method validation? Although method verification and method validation look similar, they are really not the same and have different requirements. It is important to distinguish these two terms, as they are GxP requirements to assure that the product quality is based on the guidelines of pharmaceutical industry, such as United States Pharmacopeia (USP) and International Conference on Harmonisation (ICH). To understand the difference between these two terms, let us start with the definition.
What is Analytical Method Validation?
According to the definition in USP general information chapter <1225>, “VALIDATION OF COMPENDIAL PROCEDURES”, method validation is an evaluation process on the performance characteristics of an established analytical procedure through laboratory studies with all performance characteristics meeting the intended analytical applications. In other words, an analytical method should be examined from a variety of aspects to prove that the test result of the analytical method can be trusted and appropriately applied to the intended quality objective.
For example, a tablet used to control blood pressure needs to be tested if the active pharmaceutical ingredient (API) in the tablet will be released in sufficient amount as designed. An analytical method is developed to measure the released amount of active pharmaceutical ingredient in the tablet. In this case, assay associated with dissolution may be applied as the appropriate analytical procedure to quantitate the API (or major component) in the tablet. The first priority of the established analytical method (e.g., assay and dissolution) is to validate the performance characteristics of method itself to ensure the purpose of quantitative measurement on the tablet is achieved.
Distinct aspects of the performance characteristics are required to be assessed during the method validation. There are typical analytical characteristics used for any given analytical method validation in usp general chapter <1225 verification of compendial procedures USP general chapter <1225>, “Validation of Compendial Methods” as well as ICH Q2 (R1), “Validation of Analytical Procedures: Text and Methodology”, which are demonstrated and listed as below.
- Accuracy: The accuracy of method is an evaluation of how the result is related to the true value.
- Precision: The precision of method is an assessment of repeatability on the multiple measurements, which can show how the results are distributed under normal operation.
- Specificity: The specificity of method is a performance characteristic to prove that the method is capable of identifying the desired component from the matrix components.
- Detection limit: The detection limit is a certain level (usually presented as concentration) set to determine how a method is able to distinguish the measured signal of sample from the noise of background matrix. It can be considered as an index of limit test.
- Quantification limit: The quantification limit is the lowest amount of sample (usually expressed as concentration) that can be determined by the method with acceptable precision and accuracy.
- Linearity: The linearity of method is a relationship, which reflects how the test result is proportional to the concentration of analyte in sample.
- Range: The range of method is an interval of different analyte concentration between lower and upper levels.
- Robustness: The robustness of method is an index of how a method is capable of remaining stable under normal operation despite the existence of variation from the procedures.
The analytical characteristics above are considered as data elements essential for the performance characteristics assessment.
What is Analytical Method Verification?
Method verification is an assessment focusing on how the analytical test procedure is suitable for its intended use under actual experimental condition, such as specific drug substance/product, environment, personnel, equipment, and reagent based on the definition in USP general chapter <1226>, “Verification of Compendial Procedures”. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment, laboratories to generate appropriate results for the first time.
For example, a contract manufacturing organization (CMO) laboratory has received a contract analysis request from the client to analyze Product A. An authorized test Method A from client will be performed on Product A. Now, what does this mean?
In this case:
- Method A was validated in client’s laboratory and used for analysis on Product A.
- As Method A was used in the original laboratory for analysis on Product A, method verification is required before Product A is analyzed with Method A in the CMO laboratory.
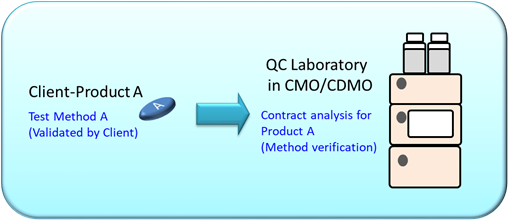
In conclusion, the laboratory must perform method verification before using Method A on Product A for the first time.
What is the Difference Between Analytical Method Verification and Analytical Method Validation?
- Method validation evaluates the performance of an established method through performing different analytical characteristics check and concludes if the method is suitable for its purpose.
- Method verification applies the necessary analytical performance characteristics as specified in the method validation to obtain reliable data for specific types of sample, environment, or equipment rather than to repeat the validation work.
For instance, a CMO receives an order from a license holder or a patent owner who has developed a new pharmaceutical product. The client will then authorize theCMO to develop the production process for a scale-up batch and also develop analytical test procedures for regulatory compliance requirement.
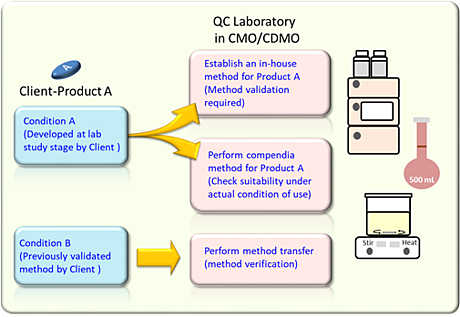
A method validation is necessary if the client has not established a proper analytical method for the regulatory requirement. The laboratory will need to establish an in-house method based on the regulatory requirement like United States Pharmacopeia (USP) and perform method validation to ensure the analytical method can meet the intended application.
On the other hand, when the laboratory performs a compendial method that have been validated with acceptable scientific evidence, there is no need to perform method validation for these compendial methods. For this case to establish suitability evidence of compendial method under actual condition of use in the laboratory is enough.
Moreover, a method verification is required if the client provides an authorized validated analytical method. The laboratory needs to perform method verification to ensure the client’s method can suitably be applied in the laboratory.
Conclusion
In conclusion, method validation is usually applied to an “in-house method” developed by a laboratory; while method verification is applied to a “compendia method or previously validated method” when it is being use in a particular laboratory for the first time. Therefore, method validation and method verification are required under different situations.
Whenever a pharmaceutical product is seeking regulatory approval, a key component of that approval includes appropriate analytical testing procedures for the drug substances and the finished product. This is a key component of the CMC section required for approval. The analytical testing methods must meet certain standards to ensure product safety and efficacy and the laboratory must be able to provide these services. If a product owner is working with a contract manufacturer, it is also essential that the CDMO/CMO maintains a laboratory with qualified equipment that is familiar with the process of method validation and method verification work, as they are a basic requirement from the regulatory authorities.
Further, it is imperative that the laboratory doing the work understands the analytical data required for regulatory approval, be it using method validation or method verification. Method validation evaluates the performance of an established method through performing different analytical characteristics. Method verification applies the necessary analytical performance characteristics as specified in the method validation to obtain reliable data for specific types of sample, environment, or equipment. If this article still creates confusion, an experienced analyst at a good CDMO/CMO laboratory can help guide you whether a method validation or method verification is needed and can then perform the proper test.