A pharmaceutical technology transfer or more commonly known as a tech transfer, is a series of knowledge transfers on a drug product and its established manufacturing processes from development to commercial production. It can also mean knowledge transfer about existing products from one manufacturing site to another by a facility change, a company merger, an acquisition, or a shift to a contract manufacturer (CMO).
A technology transfer is a complex process that involves different types of expertise. Some of those are:
- knowledge transfer
- experience sharing
- communications, and
- inter and intra-company cooperation on a large scale.
A successful tech transfer not only requires knowledge about products and processes but also knowledge about equipment, procedures, competencies, roles and responsibilities, cultures, regulatory, and training requirements.
From a pharmaceutical contract manufacturer’s (CMO) perspective, the focus is on how to deliver better results to clients from a project management point of view.
There are key considerations to lead to a successful pharmaceutical tech transfer and ultimately a product launch to market including a dedicated project management program, standardized tech transfers procedures and robust quality systems.
Project Management is Critical to the Success of a Tech Transfer
A dedicated or centralized project management team is critical in a successful product tech transfer. Initially, the project management organization or team (PMO) works closely with the business development team (BD) to understand the products/services or client needs. The PMO team then collaborates with other internal departments to fully assess the technology package. The CMO strives to understand the product and process requirements in detail and evaluates its own internal competencies to be able to successfully meet the client’s product manufacturing requirements. Once this is established, the CMO will generate a proposal and preliminary plan to successfully complete a technology transfer for the client’s product and perform routine commercial manufacturing at their site . Once the proposal is approved by the client and the project is kicked off, the project team will form a project management program to start the overall and thorough project planning before execution.
- A project manager should be assigned to coordinate the transfer activities inter and intra-companies, track the action items and milestones, monitor and control budgets, and serve as a central hub of communication between clients and CMO to ensure proper information exchange and avoid misunderstandings. Project management tools with documentation should be used to support the transfer activities.
- A project team typically consists of functional team members from the originating and receiving parties for knowledge sharing along with the transfer. The project team together performs technology package review and risk assessments. Their goal is to foresee potential issues and come up with solutions. Periodic meetings are set up for progress reporting, decision making, discussion, and experience sharing.
- Project Steering Committee and Core Team should be identified and periodic meetings should be scheduled to ensure proper communications on project progress, key decision makings, issue discussion, and risk management.
- A predefined transfer plan should be created and approved by both the originating and receiving parties to identify and align key milestones and supply guidance about transfer scope, resource requirements, timelines, and level of effort.
Standardized Tech Transfer Procedures
Contract manufacturers have to be very flexible to accommodate various client needs, a standardized tech transfer procedure and common language are established to enhance the tech transfer process.
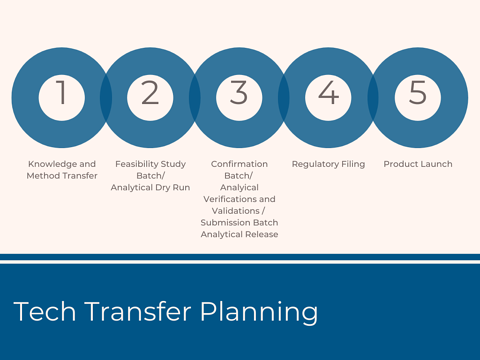
Knowledge Transfer Package and Transfer Planning
The client should provide as detailed and complete knowledge transfer package as possible with product information such as:
- raw materials
- analytical methods
- validation reports
- manufacturing procedures
- process parameters
- equipment requirements
- regulatory requirements, etc.
Contract manufacturers refer to these documents as a technology transfer package , and then they perform a thorough assessment and preparation to support the knowledge transfer process. A transfer plan is created by the Project Manager with the help of Subject Matter Experts (SMEs). The Plan is approved and executed by all the functional teams, and a kick-off meeting is held to ensure the team is aligned with the scope, strategy, and overall timeline.
Successful Process Transfer Starts With a Detailed Assessment of the Product Technical Package
A detailed assessment of the technical package must be completed to understand the scale and equipment differences in the planning stage. The Technical Team composed of such groups as technical services, manufacturing, and engineering teams perform process verification activities from small feasibility studies, small-scale, and scale-up verification runs to optimize the manufacturing process and demonstrate a successful product transfer. Protocols are created to define the study plans and data is collected to support technical reports, submission batch production, and ultimately regulatory submissions and approvals.
Analytical Method Transfers Must Begin in the Early Stages of a Tech Transfer
Analytical methods are needed in the preliminary stages of a tech transfer as all the studies require certain testing to provide data and demonstrate the study results. Raw materials that meet the specifications need to be sourced, purchased, tested and released to support study runs and process qualifications. The Analytical Team performs the following:
- raw material and finished product test method verification and validations
- create methods and specifications following regulatory requirements
- release materials and products for TS study and submission.
Regulatory Submission Preparation
Checklists are a great tool in helping to prepare regulatory submission packages by Regulatory Affairs and the functional teams.
Product Launch Preparation
The contract manufacturer should have a defined process for product launch so the team is ready for commercial supply to support the product launch. The defined process should also have a flexible and adaptable mechanism to accommodate the early or delayed approval to support the product supply for various markets following different agency policies.
Robust Quality Systems a Must!
Pharmaceutical Quality System (PQS)
A robust pharmaceutical quality system is critical to assuring drug products are manufactured to meet the desired quality and performance attributes and is the key system in providing FDA confidence that proper support information is used to make decisions. A strong and knowledgeable Quality Team who are familiar with the PQS Elements in ICH Q10 and 21 CFR 210 & 211 Components of the Quality System is key to a successful tech transfer and product launch.
Investigation and CAPA
Following 21 CFR 211.22, a Quality Control Unit shall have the responsibility and authority for the following:
- approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products,
- review production records to assure that no errors have occurred or, if errors have occurred, that they have been fully investigated.
The team needs to be capable of following proper guidelines to perform detailed investigations when in-process deviations occur. It is critical to have a defined investigation procedure and a strong team consisting of highly-trained quality engineers. Efficient and executable CAPAs should be proposed and approved by both the manufacturer and client for each confirmed deviation to properly close the investigation.
Change Control Management
The system that effectively tracks and manages changes should be used to support change controls and investigations as part of the process of technology transfer execution. The documentation of changes approved by Clients and contract manufacturer will help track and interpret discrepancies between expected and actual values. A formal system is recommended to track these changes during the transfer process.
Continuous Improvement
Continued process improvement should be planned and conducted by the technical support team in collaboration with the client to optimized product yield and bring to a more robust process control to ensure the commercial supply efficiency with desired results.
Conclusion
Technology transfer is a very common and mature process in today’s pharmaceutical industry. However, it still poses many challenges for each transfer due to different product processes, equipment design and scale, regulatory needs, communication skills, and geographic locations. Each company should set up its standardized procedure to manage technology transfers in a very structured and consistent way. A dedicated project management team is a plus to work closely with the project team to communicate and to track progress along the way to ensure timely and successful transfers.
